Glycine has pKa values of 2.34 and 9.60. At what pH does glycine exist in the forms shown?
a.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: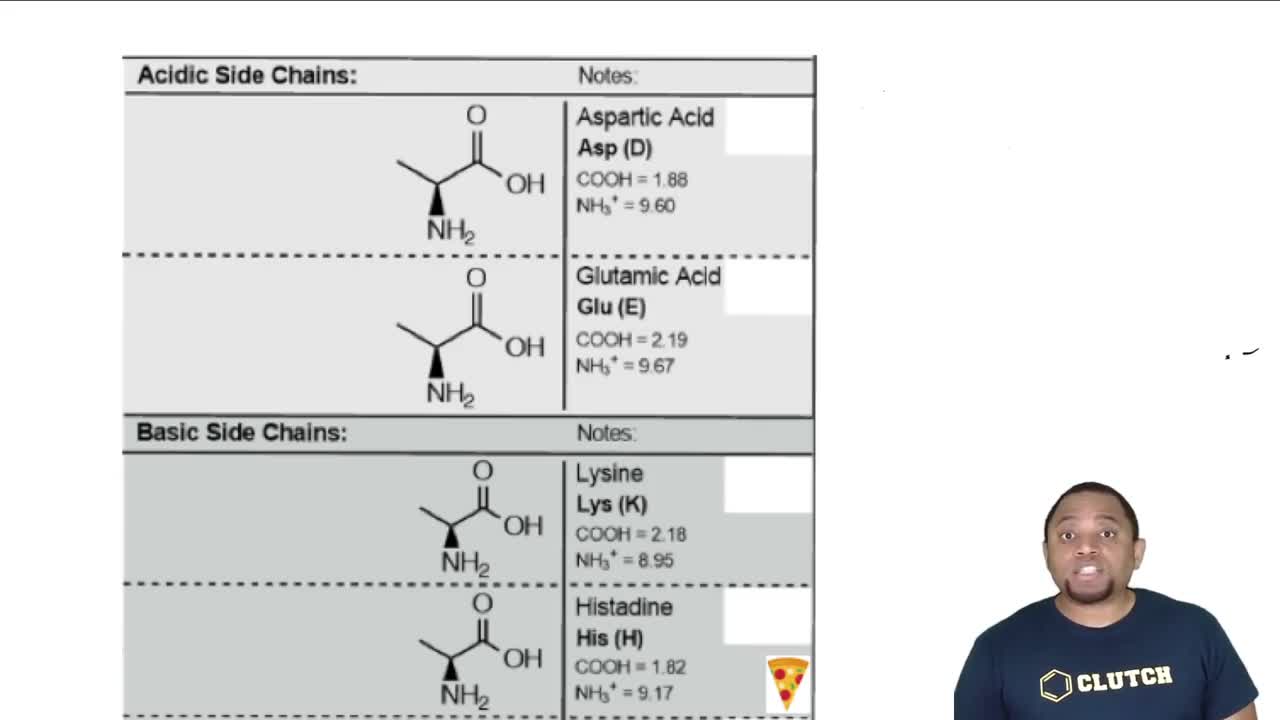
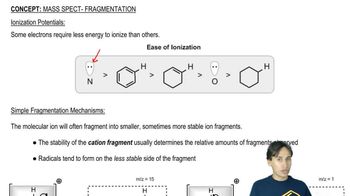
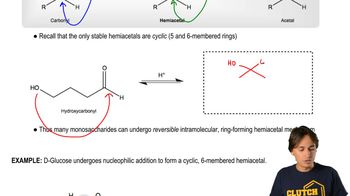

 4:41m
4:41mMaster Why Amino Acids Exist as Zwitterions with a bite sized video explanation from Johnny
Start learning