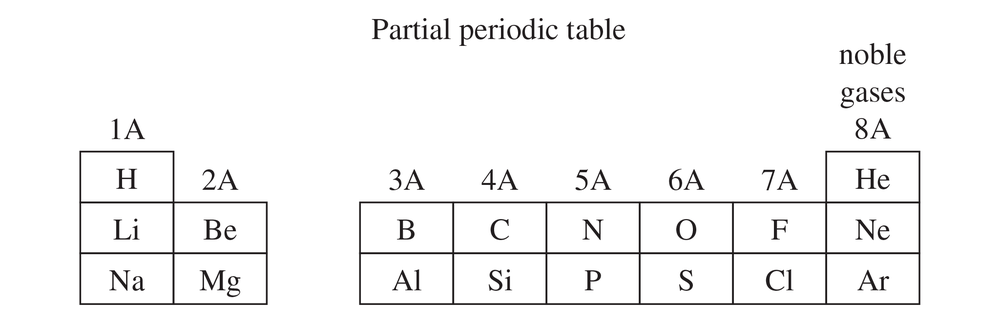
Name the element that corresponds to each electronic configuration.
a. 1s2 2s2 2p2
b. 1s2 2s2 2p4
c. 1s2 2s2 2p6 3s2 3p3
d. 1s2 2s2 2p6 3s2 3p5
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:44m
1:44mMaster The difference between atomic numbers and atomic mass. with a bite sized video explanation from Johnny
Start learning