a. How many protons do the following species have?
b. How many electrons does each have?
- Na+
- Ar
- Cl-
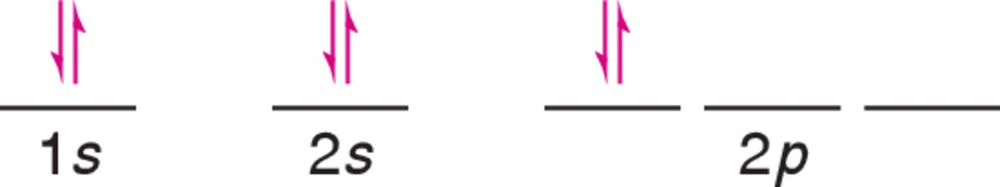
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:44m
1:44mMaster The difference between atomic numbers and atomic mass. with a bite sized video explanation from Johnny
Start learning