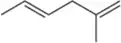
What is the index of hydrogen deficiency for each of the following molecular formulas?
(d) C9H12N2O2
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: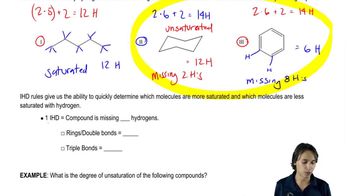

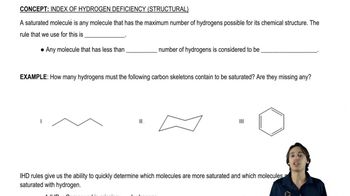

 2:39m
2:39mMaster The difference between saturated and unsaturated molecules. with a bite sized video explanation from Johnny
Start learning