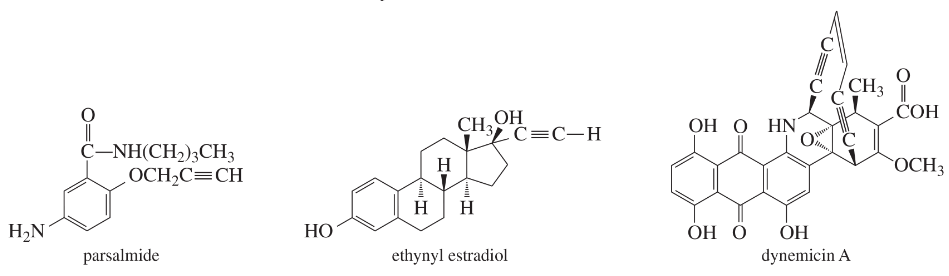
Given the structure, calculate the index of hydrogen deficiency (degrees of unsaturation) of the following molecules.
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 2:39m
2:39mMaster The difference between saturated and unsaturated molecules. with a bite sized video explanation from Johnny
Start learning