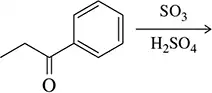
Are the following substituents ortho–para directors or meta directors?
a. CH=CHC≡N
b. NO2
c. CH2OH
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
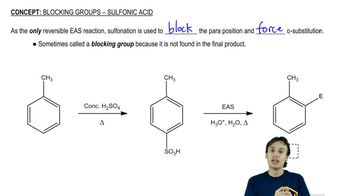
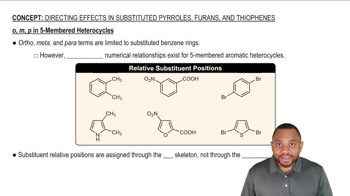

 4:29m
4:29mMaster Activity and Directing Effects with a bite sized video explanation from Johnny
Start learning