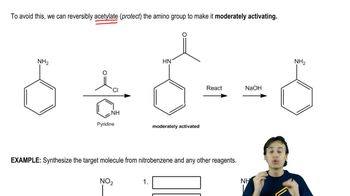
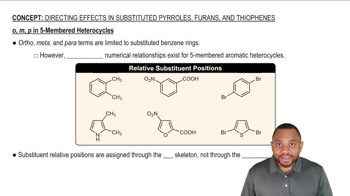
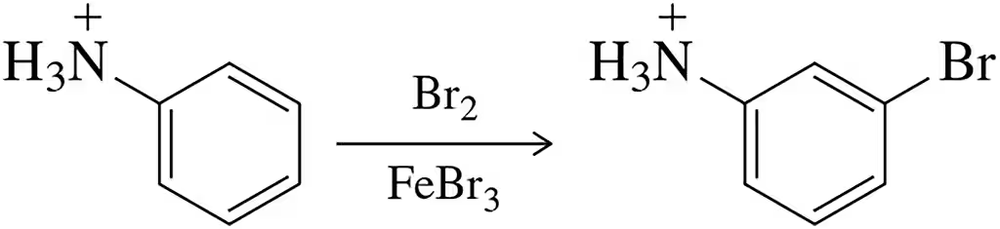Predict the major product(s) that would result when molecules (a)–(i) are allowed to react under the following conditions. (iv) chlorocyclopentane, AlCl3, (v) 1-chloro-1-methylcyclohexane , AlCl3, (vi) PhCOCl, AlCl3. If no reaction will occur, indicate by writing NR.
(a)