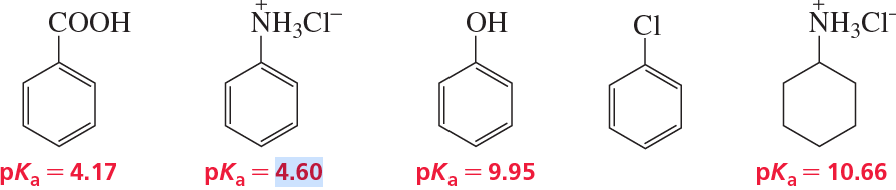
Write equations for the following acid–base reactions. Use the information in Table 2-2 or Appendix 4 to predict whether the equilibrium will favor the reactants or the products.
a. HCOOH + –CN
b. CH3COO– + CH3OH
c. (CH3)2CHOH + NaNH2
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 5:11m
5:11mMaster The 3 steps for determining the direction of acid and base equilibrium. with a bite sized video explanation from Johnny
Start learning