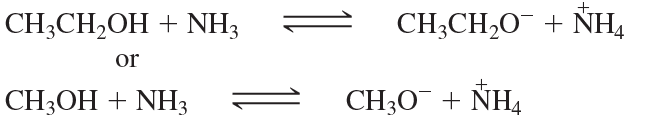
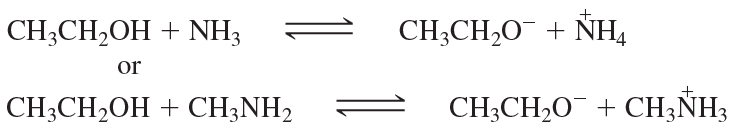
Consider the following proposed Brønsted–Lowry acid–base reactions. In each case, draw the products of a transfer of the most acidic proton on the acid to the most basic site on the base. Use Appendix 4 to find or estimate the pKa values for the acids and the pKb values for the bases. Then determine which side of the reaction is favored, either reactants or products.
(a)
(b)