Draw orbital pictures of the pi bonding in the following compounds:
e. CH3CH=C=CHCH3
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: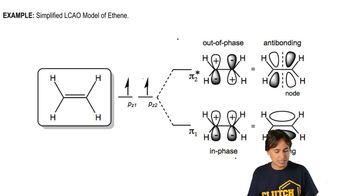
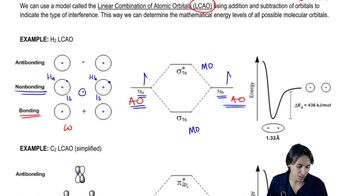

 2:19m
2:19mMaster What’s the difference between sigma and pi bonds? with a bite sized video explanation from Johnny
Start learning