Multiple Choice
Calculate the pI for arginine (the pKa values—or the pKa values for the conjugate acids of bases—are given).
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: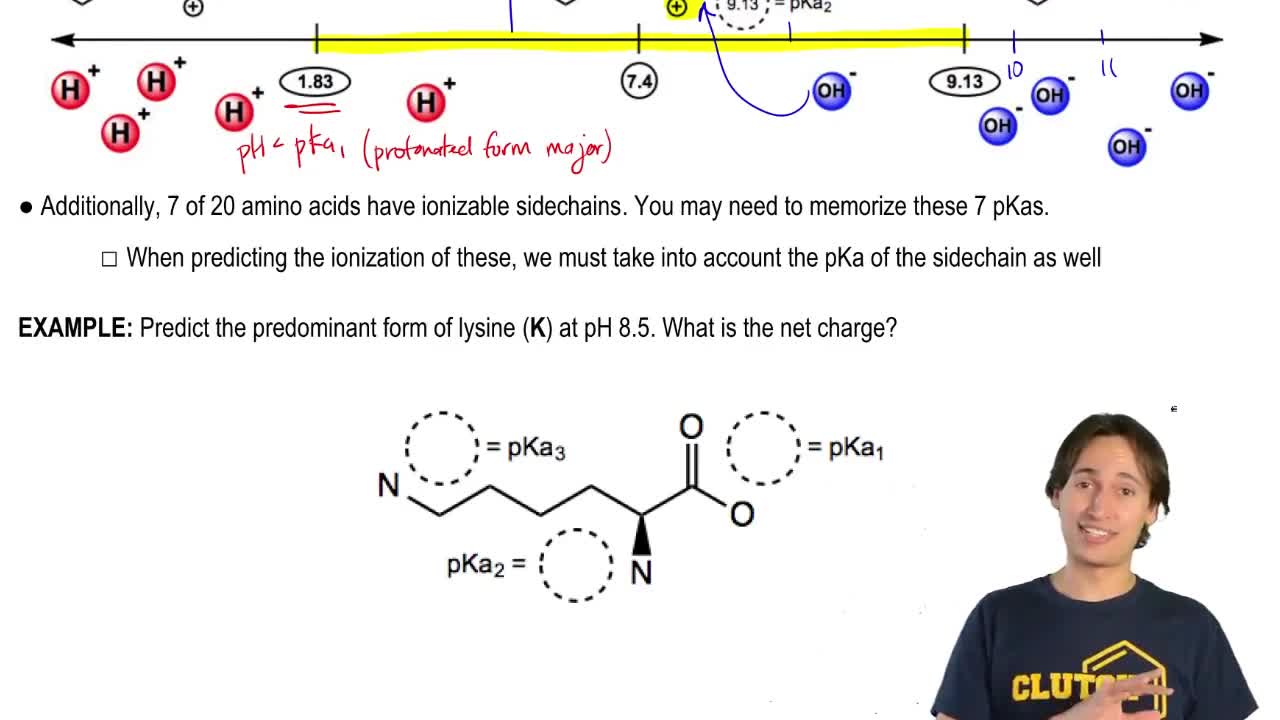


 4:41m
4:41mMaster Why Amino Acids Exist as Zwitterions with a bite sized video explanation from Johnny
Start learning