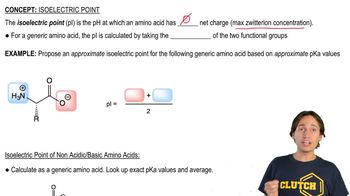
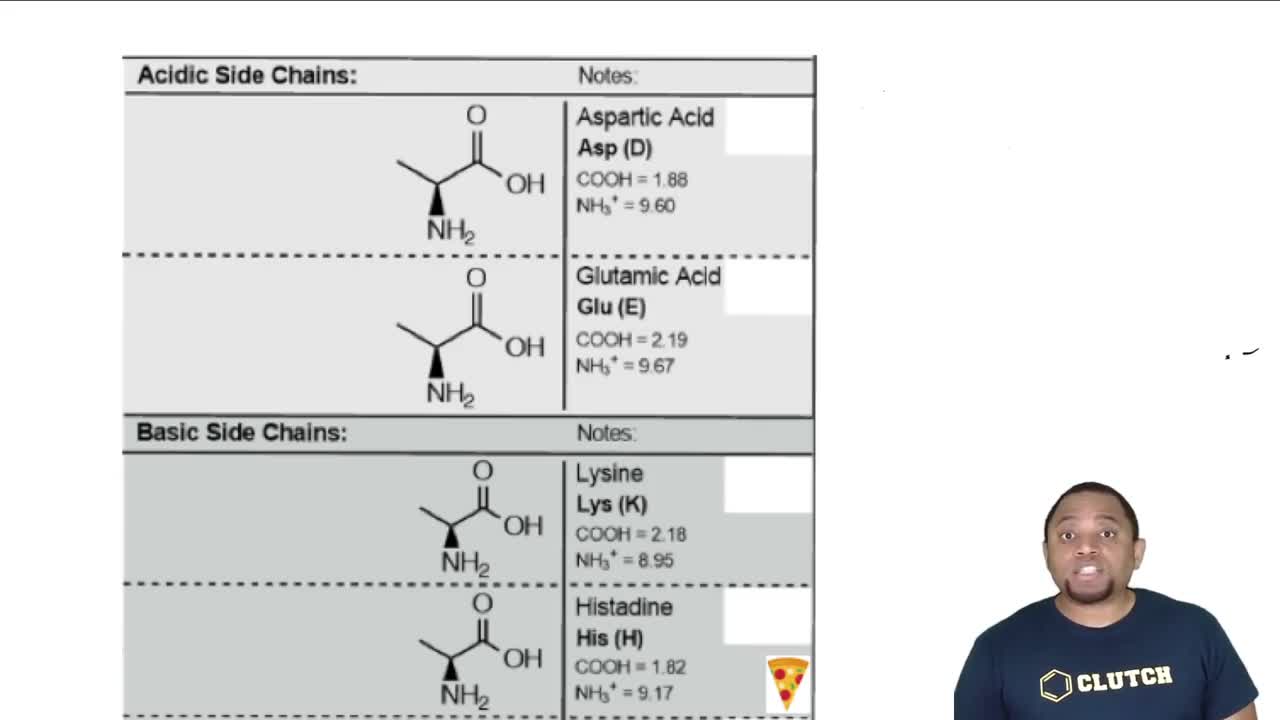
Most naturally occurring amino acids have chiral centers (the asymmetric α carbon atoms) that are named (S) by the Cahn–Ingold–Prelog convention (Section 5-3). The common naturally occurring form of cysteine has a chiral center that is named (R), however.
(b) (S)-Alanine is an L-amino acid (Figure 24-2). Is (R)-cysteine a d-amino acid or an L-amino acid?