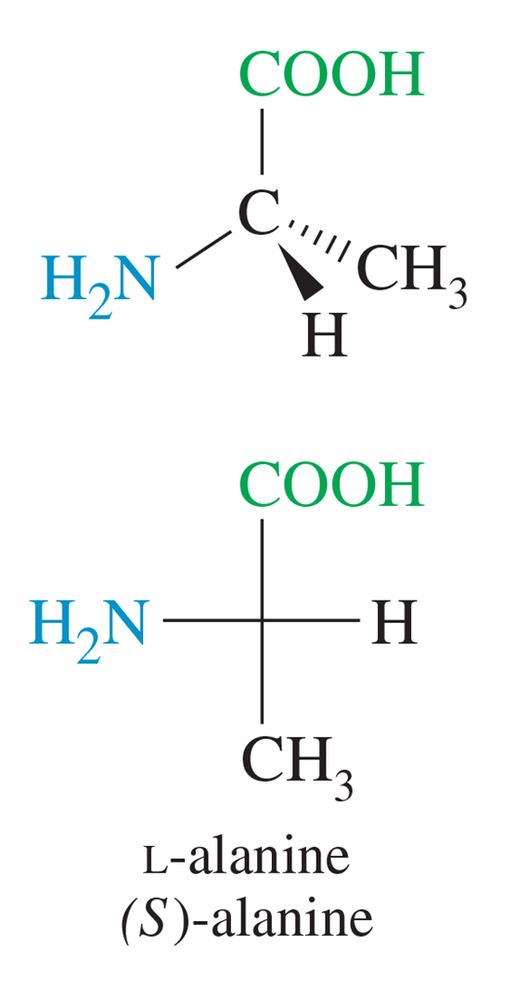
a. What percentage of the a-amino group of lysine will be protonated at its pI?
<25%, 50%, >75%
b. Answer the same question for the e-amino group of lysine
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:19m
3:19mMaster Definition of Isoelectric Point with a bite sized video explanation from Johnny
Start learning