a. Which amino acid has the lowest pI value?
b. Which amino acid has the highest pI value?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: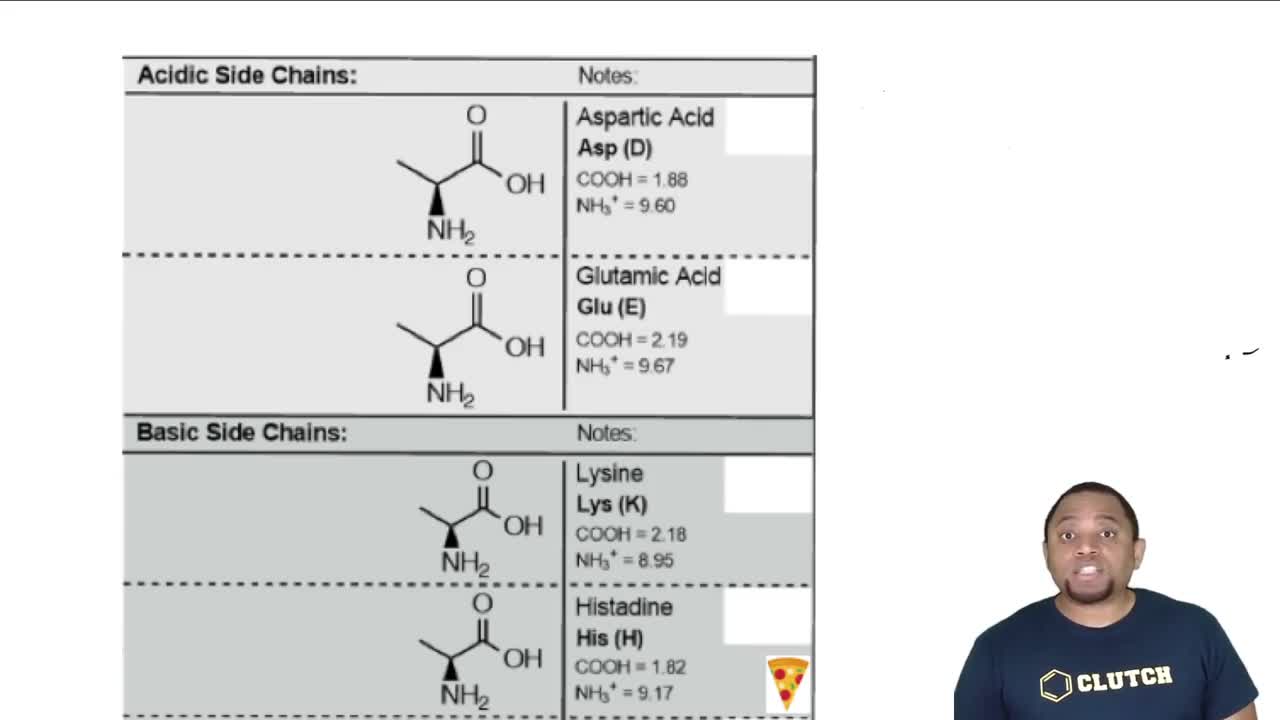


 3:19m
3:19mMaster Definition of Isoelectric Point with a bite sized video explanation from Johnny
Start learning