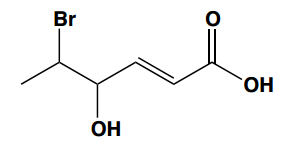
The following carboxylic acids were named incorrectly. Provide the correct name.
(b) 6-bromocyclohexane carboxylic acid
 Verified step by step guidance
Verified step by step guidance
 4:20m
4:20mMaster Carboxylic Acids Nomenclature with a bite sized video explanation from Johnny
Start learning
