Show how you would accomplish the following conversions.
d. trans-hex-3-ene to (d,l)-hexane-3,4-diol
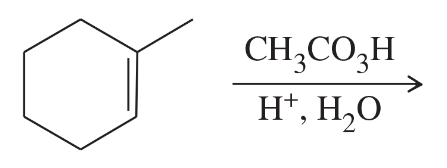
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:50m
3:50mMaster General properties of syn vicinal dihydroxylation. with a bite sized video explanation from Johnny
Start learning