The two butenedioic acids are called fumaric acid (trans) and maleic acid (cis). 2,3-Dihydroxybutanedioic acid is called tartaric acid.
Show how you would convert
c. maleic acid to (±)-tartaric acid.
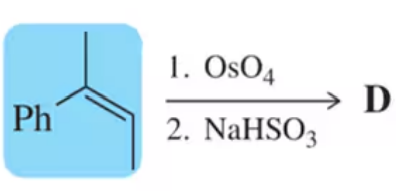
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:50m
3:50mMaster General properties of syn vicinal dihydroxylation. with a bite sized video explanation from Johnny
Start learning