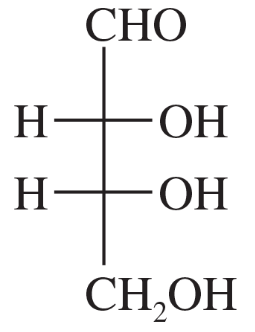
Which of the following compounds are chiral? Draw each compound in its most symmetric conformation, star (*) any asymmetric carbon atoms, and draw any mirror planes. Label any meso compounds. You may use Fischer projections if you prefer.
(e) (R,R)-2,3-dibromobutane
(f)