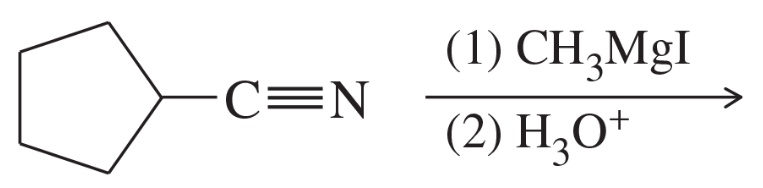There are many methods for activating a carboxylic acid in preparation for coupling with an amine. The following method converts the acid to an N-hydroxysuccinimide (NHS) ester.
(c) Propose a mechanism for the reaction of the NHS ester with an amine, R–NH2.