There are many methods for activating a carboxylic acid in preparation for coupling with an amine. The following method converts the acid to an N-hydroxysuccinimide (NHS) ester.
(b) Propose a mechanism for the reaction shown.
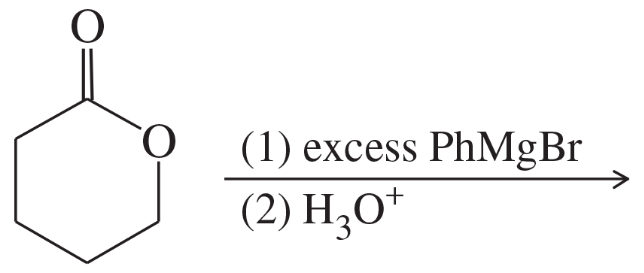
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 9:32m
9:32mMaster NAS - The Three Rules with a bite sized video explanation from Johnny
Start learning