A molecular orbital diagram is shown for the C―Cl bond in chloromethane. If two more electrons were added to chloromethane, where would the electrons go?
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: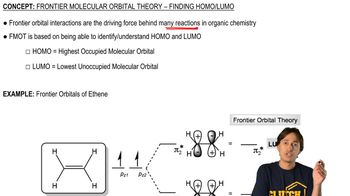

 2:19m
2:19mMaster What’s the difference between sigma and pi bonds? with a bite sized video explanation from Johnny
Start learning