Textbook Question
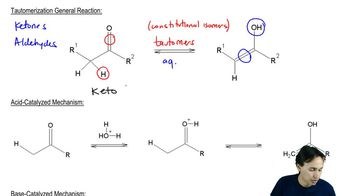
Cytosine, uracil, and guanine have tautomeric forms with aromatic hydroxy groups. Draw these tautomeric forms.
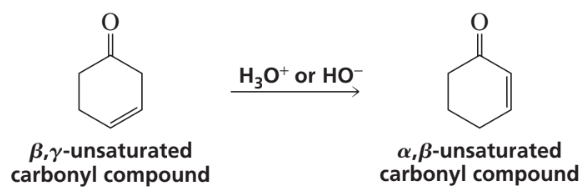
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:51m
1:51mMaster Unusual Acidity of the Alpha Carbon with a bite sized video explanation from Johnny
Start learning