Phenylacetone can form two different enols.
(c) Propose mechanisms for the formation of the first enol in base.
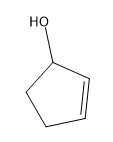

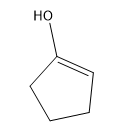
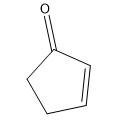
 Verified step by step guidance
Verified step by step guidance
 1:51m
1:51mMaster Unusual Acidity of the Alpha Carbon with a bite sized video explanation from Johnny
Start learning
