Open Question
Draw the structures of the compounds A and B for the following synthesis.
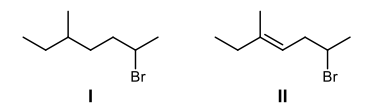
I
II
Both will proceed at equal rates.
 Verified step by step guidance
Verified step by step guidance
 1:26m
1:26mMaster Reactions at the Allylic Position Concept 1 with a bite sized video explanation from Johnny
Start learning
