Multiple Choice
Which of the following alcohols will undergo acid-catalyzed dehydration at the fastest rate?
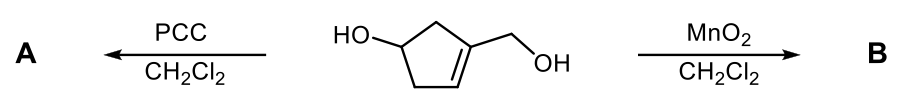
 Verified step by step guidance
Verified step by step guidance
 1:26m
1:26mMaster Reactions at the Allylic Position Concept 1 with a bite sized video explanation from Johnny
Start learning
