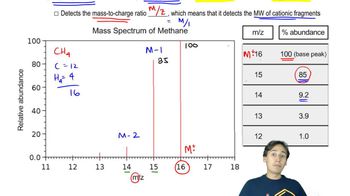
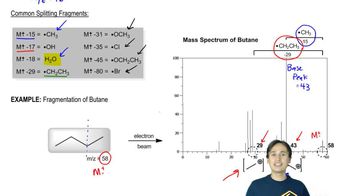
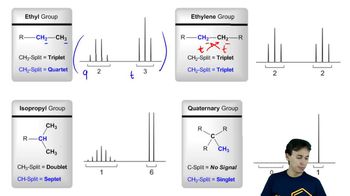
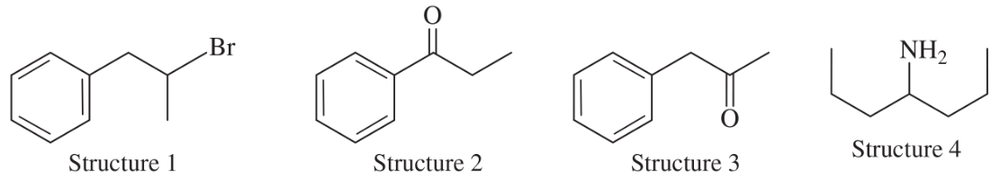
Besides the molecular mass, how do you know that the fragment that gives rise to the peak at m/z 111 in the mass spectrum for 1-bromo-4-chlorobenzene contains only chlorine?
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: