Show a reaction coordinate diagram for the two processes in Figure 13.41 that rationalizes pathway B as the one that gives the major product.
<IMAGE>
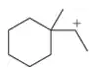
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
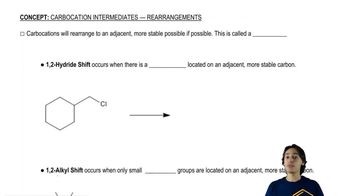
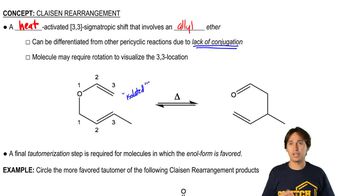

 0:47m
0:47mMaster Understanding why carbocations shift. with a bite sized video explanation from Johnny
Start learning