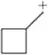
The molecular orbital picture of H2 can be represented by the following diagram. Label σ and σ* on the diagram—that is, which is (a) and which is (b)? Which is lower in energy? Why?
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 0:47m
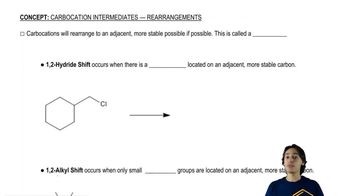
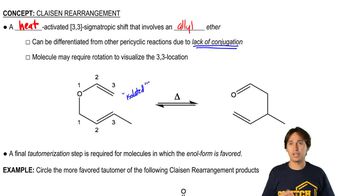
0:47mMaster Understanding why carbocations shift. with a bite sized video explanation from Johnny
Start learning