Convert each Newman projection to the equivalent line–angle formula, and assign the IUPAC name.
(a)
(b)
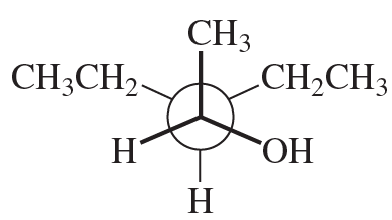
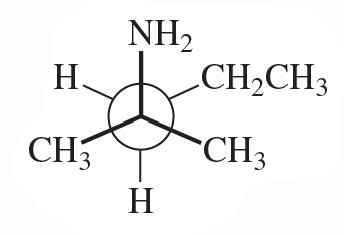
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:34m
0:34mMaster Introduction to Drawing Newman Projections with a bite sized video explanation from Johnny
Start learning