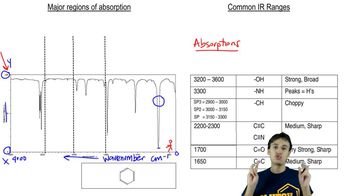
Identify the peaks in the mass spectrum of octan-4-one that correspond to (a) α-cleavage.
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: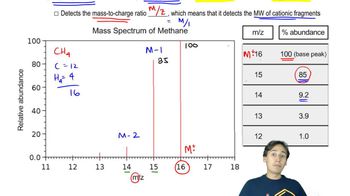


 4:28m
4:28mMaster Ionization Potentials with a bite sized video explanation from Johnny
Start learning