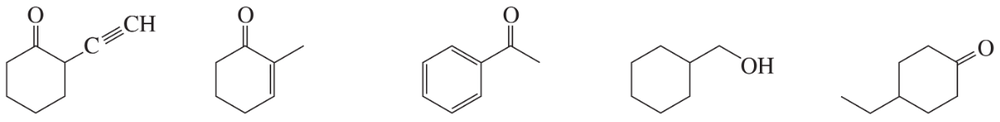
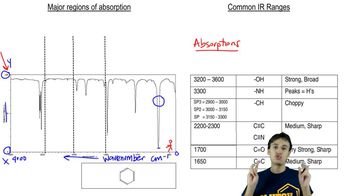
On a day in which the laboratory air conditioning stopped working, a chemist hypothesized that the atmosphere would provide enough ambient heat to drive the nitrile hydrolysis reaction to the carboxylic acid. Based on the IR of the resulting product, was this hypothesis correct?
<IMAGE>