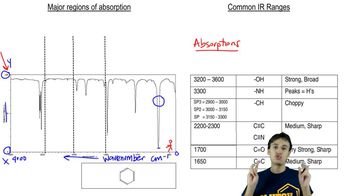
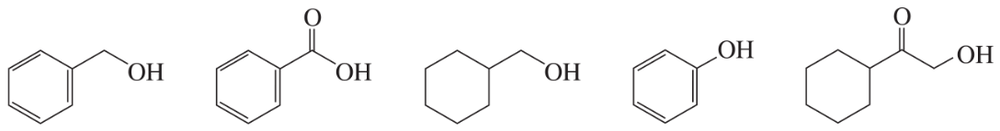
Hoping to make the following diene, a chemist treated the diol shown with acid. Based on the IR spectrum, was the reaction successful? If not, what compound was made instead?
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: