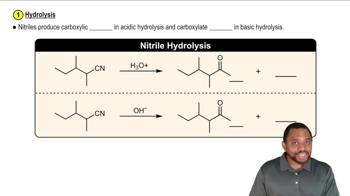
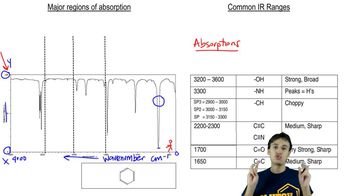
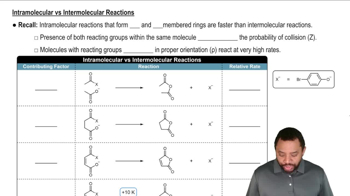
What functional groups might be present in the IR spectra for the molecules with the given molecular formulas. [Be sure to use the molecular formula in your analysis.]
(b) C₃H₄O₄
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: