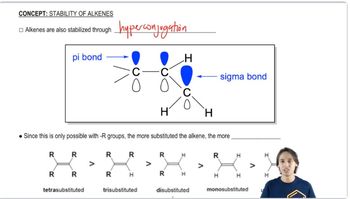
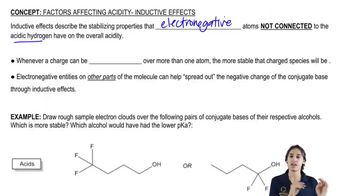
For each pair of reactions, predict which will happen more quickly. [For (c) and (d), the more substituted carbocation is more stable due to hyperconjugation.]
(c)

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 5:58m
5:58mMaster Determining Carbocation Stability with a bite sized video explanation from Johnny
Start learning