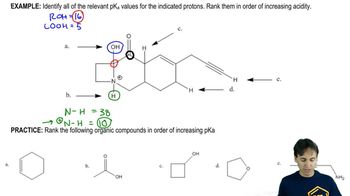
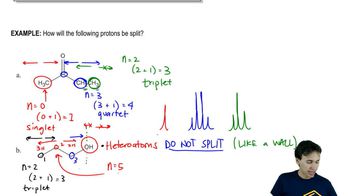
In the following reactions,
(i) identify the acid and base,
(ii) identify the most electron-rich atom in the base,
(iii) identify the most acidic hydrogen in the acid,
(iv) provide an arrow-pushing mechanism of the proton transfer that will occur, and
(v) predict the product of the reactions. [You'll need to provide the lone pairs here.]
(d) H2O + HCl ⇌