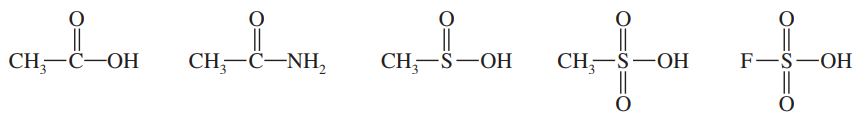A naturally occurring amino acid such as alanine has a group that is a carboxylic acid and a group that is a protonated amine. The pKa values of the two groups are shown.
e. Is there a pH at which alanine is uncharged (that is, neither group has a charge)?
f. At what pH does alanine have no net charge (that is, the amount of negative charge is the same as the amount of positive charge)?