Predict the product of the following electrocyclic reactions.
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: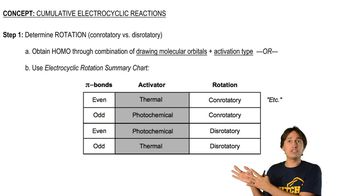
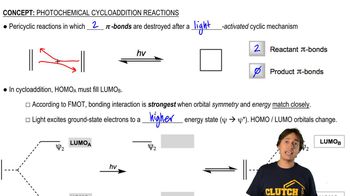
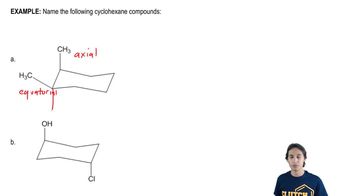

 4:49m
4:49mMaster MO Theory of Photochemical Electrocyclics with a bite sized video explanation from Johnny
Start learning