Label each set of chemically equivalent protons, using a for the set that will be at the lowest frequency in the 1H NMR spectrum, b for the next lowest, and so on. Indicate the multiplicity of each signal.
a.
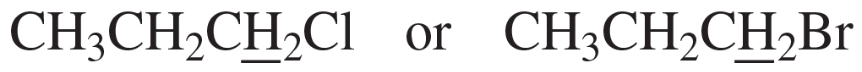
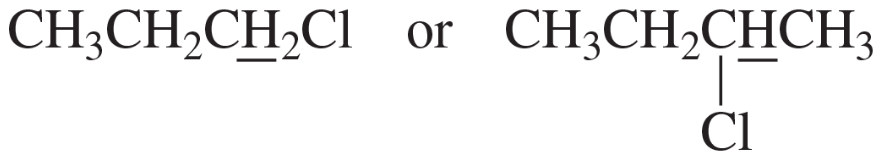
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 11:44m
11:44mMaster 1H NMR Chemical Shifts with a bite sized video explanation from Johnny
Start learning