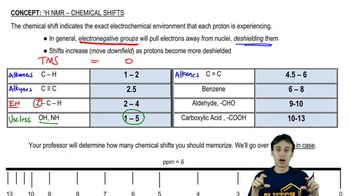
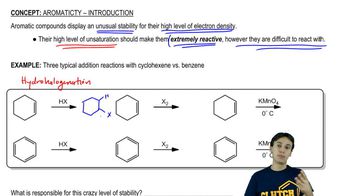
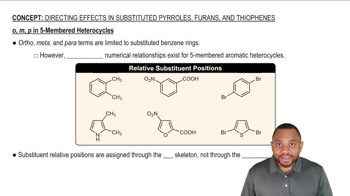
In a 300-MHz spectrometer, the protons in iodomethane absorb at a position 650 Hz downfield from TMS.
(a) What is the chemical shift of these protons?
(b) What is the chemical shift of the iodomethane protons in a 60-MHz spectrometer?
(c) How many hertz downfield from TMS would they absorb at 60 MHz?