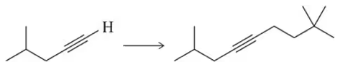
Calculate Keq for the following acid–base reactions. Which is the best base to use to deprotonate acetylene and make the acetylide anion?
(a) HO- + H―C ≡ C―H ⇌ -C ≡ C―H + H2O
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: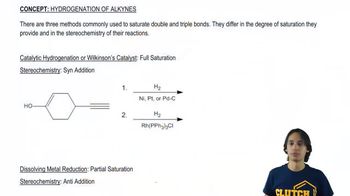


 4:19m
4:19mMaster Understanding how to convert terminal alkynes to alkynides. with a bite sized video explanation from Johnny
Start learning