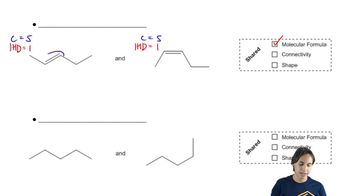
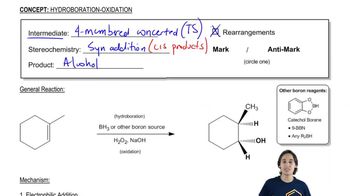
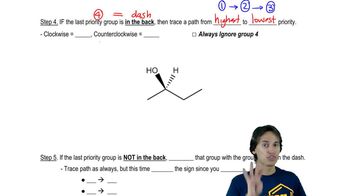
Predict the products you would get when the following alkenes undergo (i) hydroboration–oxidation (1. BH3 2. NaOH, H2O2 or (ii) oxymercuration–reduction [1. Hg(OAc)2, H2O 2. NaBH4].
(a)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: