Suggest an alkene to undergo hydroboration–oxidation (1. BH3 2. NaOH, H2O2) to give exclusively the alcohols shown. Pay close attention to the relative (but not absolute) stereochemical outcome.
(c)
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: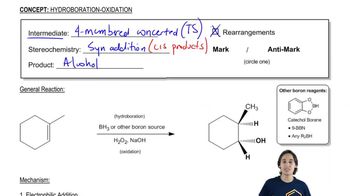


 6:38m
6:38mMaster General properties of hydroboration-oxidation. with a bite sized video explanation from Johnny
Start learning