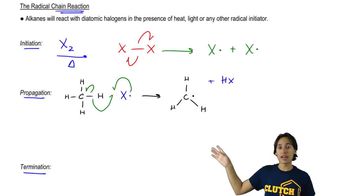
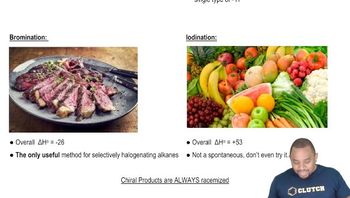
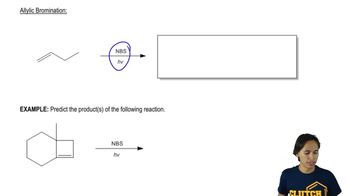
Textbook Question
Show how free-radical halogenation might be used to synthesize the following compounds. In each case, explain why we expect to get a single major product.
(c)
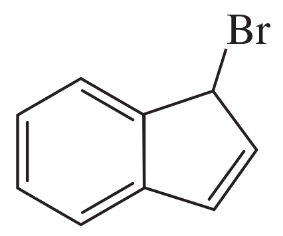
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: