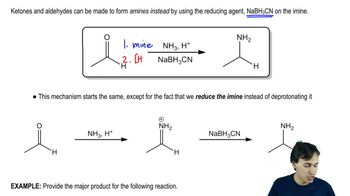
The two most general amine syntheses are the reductive amination of carbonyl compounds and the reduction of amides. Show how these techniques can be used to accomplish the following syntheses.
(b) benzaldehyde → benzylamine
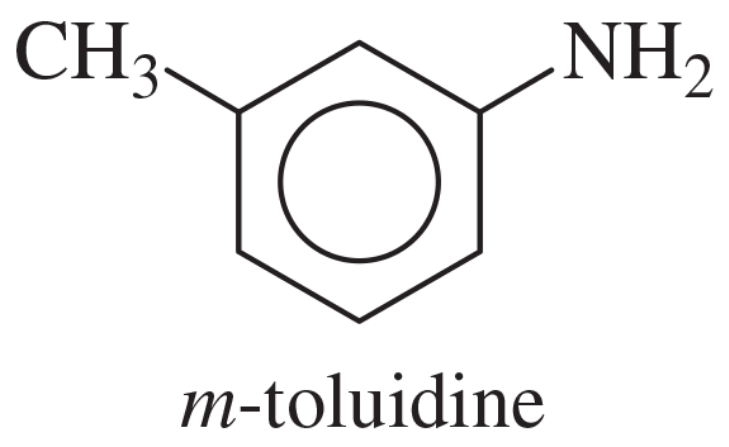
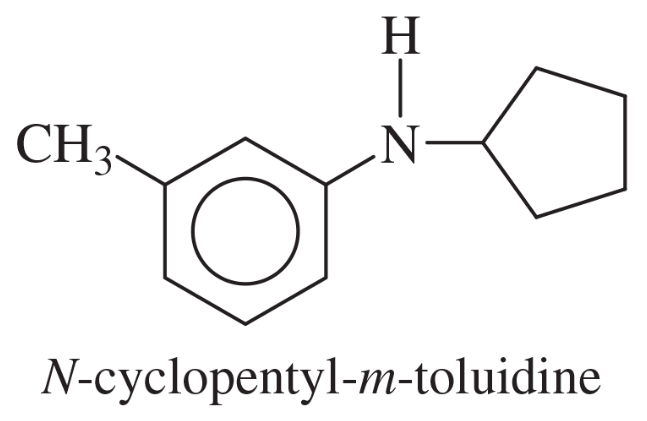
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: