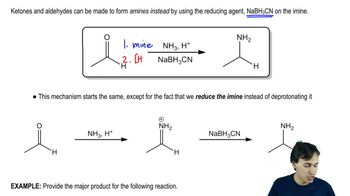
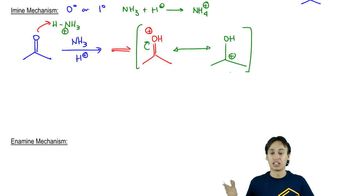
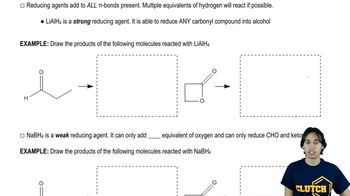
The compounds commonly known as 'amino acids' are actually α-aminocarboxylic-aminocarboxylic acids. What carbonyl compounds should be used to synthesize the two amino acids shown here?
b.
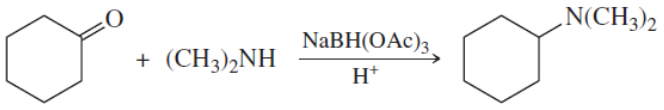
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: