Represent each of the following condensed structural formulas using a line-angle drawing.
(d) CH3CH2CH2CCH2OH
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: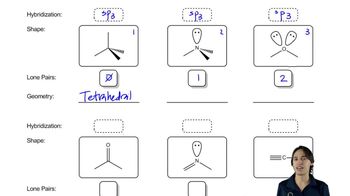

 6:06m
6:06mMaster How to interpret condensed structures. with a bite sized video explanation from Johnny
Start learning