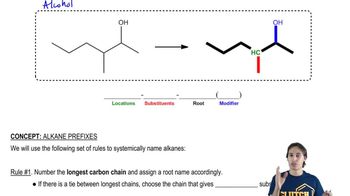
Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description.
e. a (2,3-dimethylpentyl)cycloalkane
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 1:11m
1:11mMaster How to find the root name for cycloalkanes with a bite sized video explanation from Johnny
Start learning