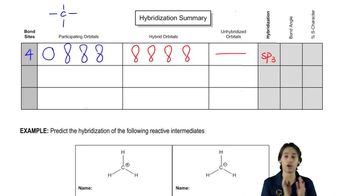
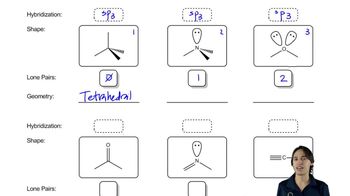
For the following partial structures, the σ bond is shown. Add the indicated number of π bonds, being sure to specify the orientation (that is, x, y, or z axis) of the p orbitals used.
(e)
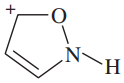
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: