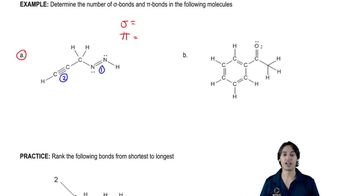
In addition to radicals, anions, and cations, a fourth class of reactive intermediates is carbenes. A neutral species, the simplest carbene has a molecular formula of CH2.
(b) What are the hybridization and shape of the central carbon of CH2?
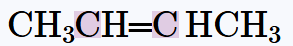
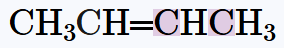
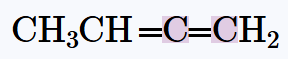
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:53m
2:53mMaster How carbon creates 4 partially-filled orbitals. with a bite sized video explanation from Johnny
Start learning