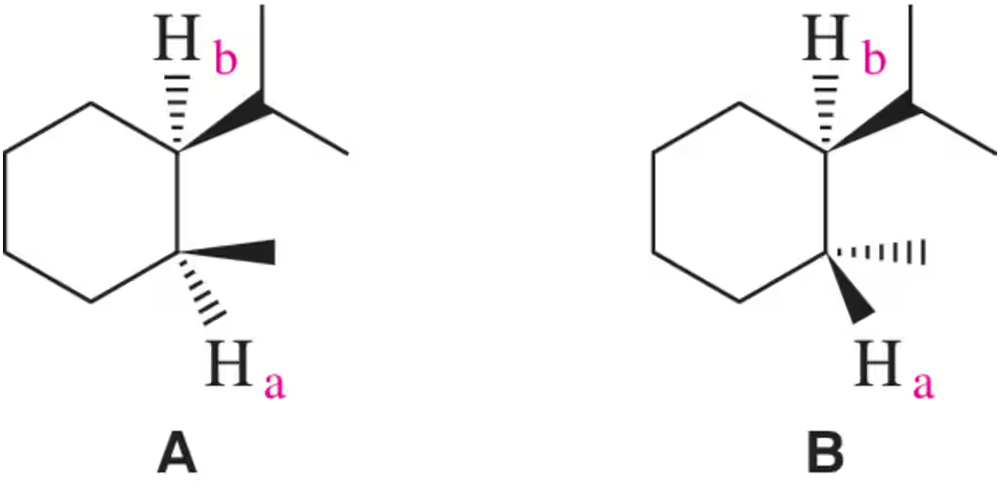
Draw a diagram like the one shown in Figure 14.12 to predict
a. the relative intensities of the peaks in a triplet.
<IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 11:3m
11:3mMaster Splitting with J-Values:Simple Tree Diagram with a bite sized video explanation from Johnny
Start learning